
When two or more atoms of different elements combine together then it is called a compound. The positive metals and negative electrons stick together in a fairly organised lattice structure. When two or more atoms join together chemically they form a molecule. For example, two atoms of hydrogen hook together to form a molecule of hydrogen. The extra electrons are now free and form a sea of electrons, which is negatively charged. Atoms can join together - they form bonds together - to make MOLECULES. Metallic bonding is when two metals lose their extra electrons and become positive ions.
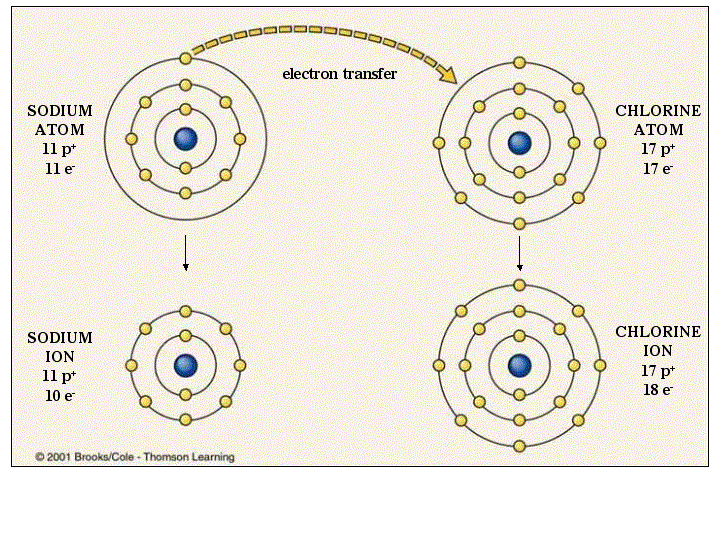
An example of this is in table salt, #NaCl#, sodium chloride. Like North and South on a magnet, the negative and positive ions stick together. Since electrons are negatively charged, this makes the non-metal a negative ion, and the metal a positive ion. Ionic bonding is when a metal atom gives electrons to a non-metal.
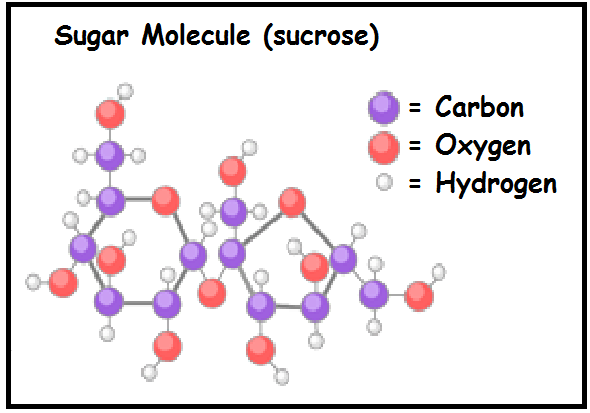
Covalent bonds only occur with two non-metals, like in water, #H_2O#.
WHEN ATOMS JOIN TOGETHER THEY FORM FULL
When they share or give electrons, the two atoms have a bond between them and stick together, unless you give them loads of energy to break the bond.Ĭovalent bonding is when two atoms share the outer electrons, so that both have a full outer shell. To do this, two or more atoms will share or give away electrons to each other in a process called bonding. The inner shell can only hold #2# electrons, and all the shells after that hold #8#, except in transition metals and really big atoms.įor an atom to become totally stable, it needs to have a full outer shell. Electrons are organised in shells, or energy levels, and each shell has a certain capacity. Atoms consist of a nucleus made of protons and neutrons in the center, and electrons orbiting around the outside.


 0 kommentar(er)
0 kommentar(er)
